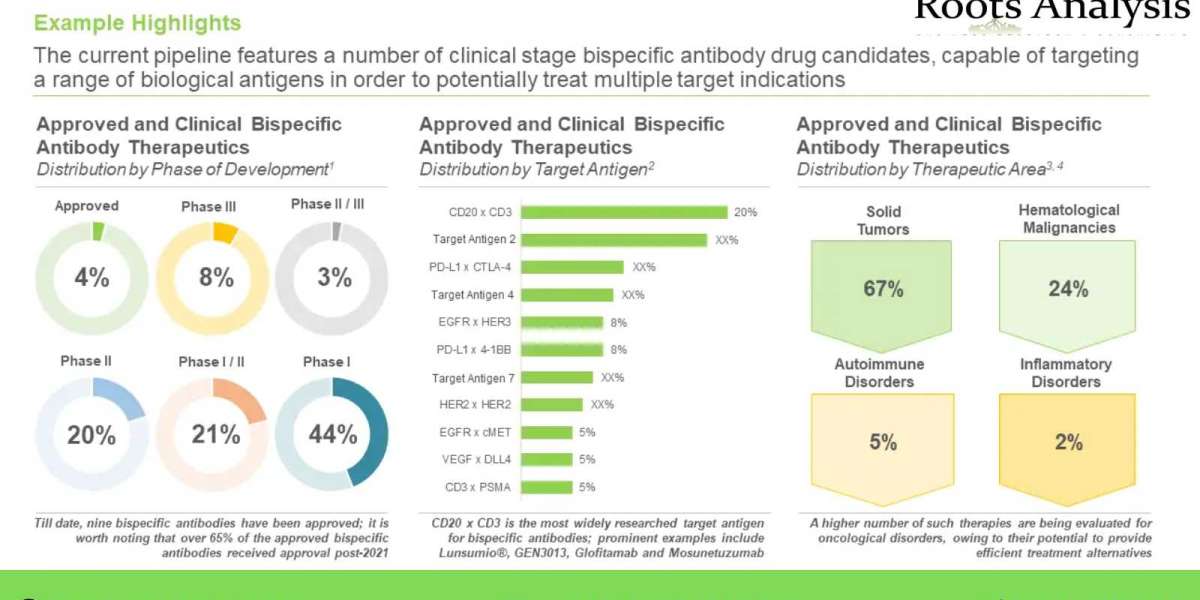
Key Market Insights
- Presently, over 220 candidates are currently being evaluated across different stages of clinical development and nine bispecific antibody therapeutics have been commercialized
- Over 180 candidates are currently under preclinical studies and over 30% of such candidates are currently being evaluated under IND studies
- More than 120 players currently claim to be engaged in the bispecific antibody therapeutics domain
- Over 70 bispecific antibody therapeutics are being developed by big pharma players, across the globe
- Nearly 35% of CAGR was witnessed in the partnership activity in this market, in the past seven years
- More than 37,000 patients have been enrolled in close to 280 clinical trials, being conducted across the globe
- Over 60% of the projected bispecific antibody market opportunity in this domain, in 2035, is anticipated to be captured by players based in North America
Table of Contents
- PREFACE
1.1. Introduction
1.2. Key Market Insights
1.3. Scope of the Report
1.4. Research Methodology
1.5. Frequently Asked Questions
1.6. Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
3.1. Chapter Overview
3.2. Introduction to Antibodies
3.3. Antibody Therapeutics
3.4. Bispecific Antibody Formats
3.5. Mechanism of Action of Bispecific Antibody Therapeutics
Other Mechanisms
3.6. Applications of Bispecific Antibodies
- MARKET LANDSCAPE OF APPROVED AND CLINICAL THERAPIES
4.1. Chapter Overview
4.2. Approved and Clinical Bispecific Antibody Therapeutics: Overall Market Landscape
4.3. Approved and Clinical Bispecific Antibody Therapeutics: Overall Developer Landscape
- MARKET LANDSCAPE OF PRECLINICAL THERAPIES
5.1. Chapter Overview
5.2. Preclinical Bispecific Antibody Therapeutics: Overall Market Landscape
5.3. Preclinical Bispecific Antibody Therapeutics: Overall Developer Landscape
- KEY INSIGHTS
6.1. Chapter Overview
6.2. Approved and Clinical Stage Bispecific Antibody Therapeutics Landscape: Key Insights
6.3. Preclinical Stage Bispecific Antibody Therapeutics Landscape: Key Insights
6.4. Bispecific Antibody Therapeutic Developers Landscape: Key Insights
- TECHNOLOGY ASSESSMENT FRAMEWORK
7.1. Chapter Overview
7.2. Bispecific Antibody Therapeutics: List of Technology Platforms
7.3. Bispecific Antibody Technology Platforms: Comparative Analysis
- BIG PHARMA PLAYERS: BENCHMARKING ANALYSIS
8.1. Chapter Overview
8.2. Big Pharma Players: Benchmarking Analysis (Spider Web Representation)
8.3. Big Pharma Players: Comparative Clinical Pipeline Analysis
- COMPANY PROFILES
9.1. Chapter Overview
9.2. Akeso Biopharma
9.2.1. Company Overview
9.2.2. Financial Performance
9.2.3. Drug Portfolio
9.2.4. Recent Developments and Future Outlook
9.3. Alphamab Oncology
9.4. Amgen
9.5. Merck
9.6. Regeneron
9.7. Roche
9.8. Xencor
- PARTNERSHIPS AND COLLABORATIONS
10.1. Chapter Overview
10.2. Partnership Models
10.3. Bispecific Antibody Therapeutics: Partnerships and Collaborations
- CONTRACT SERVICES FOR BISPECIFIC ANTIBODY THERAPEUTICS
11.1. Chapter Overview
11.2. Manufacturing of Bispecific Antibody Therapeutics
11.3. Key Considerations for Selecting a Suitable CMO / CRO Partner
- CLINICAL TRIAL ANALYSIS
12.1. Chapter Overview
12.2. Key Assumptions and Methodology
12.3. Bispecific Antibody Therapeutics: Clinical Trial Analysis
- CASE STUDY: REGULATORY GUIDELINES FOR BISPECIFIC ANTIBODIES
13.1. Chapter Overview
13.2. Guidelines Issued by Regulatory Authorities
- CASE STUDY: PROMOTIONAL / MARKETING STRATEGIES
14.1. Chapter Overview
14.2. Key Channels Used for Promotional Campaigns
14.3. Other Channels Used for Promotional and Marketing Strategies
14.4. Summary: Promotional and Marketing Strategy Leveraged by Bispecific Antibody Developers
14.5. Promotional Analysis: Blincyto™
14.6. Promotional Analysis: Hemlibra®
- MARKET FORECAST AND OPPORTUNITY ANALYSIS
15.1. Chapter Overview
15.2. Scope and Limitations
15.3. Forecast Methodology and Key Assumptions
15.4. Bispecific Antibody Therapeutics Market, 2023-2035
15.5. Bispecific Antibody Market: Value Creation Analysis
15.6. Bispecific Antibody Therapeutics Market: Product-wise Sales Forecasts
15.7. Concluding Remarks
- SWOT ANALYSIS
16.1. Chapter Overview
16.2. Strengths
16.3. Weaknesses
16.4. Opportunities
16.5. Threats
16.6. Concluding Remarks
- CONCLUDING REMARKS
- EXECUTIVE INSIGHTS
18.1. Chapter Overview
18.2. Immunai
18.3. University of Freiburg
18.4. SYNIMMUNE
18.5. CytomX Therapeutics
18.6. F-star
18.7. Innovent Biologics
- APPENDIX 1: TABULATED DATA
- APPENDIX 2: LIST OF COMPANIES AND ORGANIZATION
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/view_document/bispecific-antibodies/286.html
Learn from experts: do you know about these emerging industry trends?
Top 10 Cell and Gene Therapy Companies
Top 5 Companies Developing Bispecific Antibodies
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Learn more about Roots Analysis consulting services:
Contact:
Ben Johnson
+1 (415) 800 3415